Abstract
Background: Sickle cell centers and pediatricians often work together to ensure appropriate vaccination of patients with sickle cell disease (SCD). Patients with SCD are at high risk for serious infections with encapsulated bacteria, such as Neisseria menigitidis and Streptococcus pneumoniae; thus, vaccine recommendations for pediatric patients with SCD differ from those for the general population. The University of Alabama at Birmingham Lifespan Comprehensive Sickle Cell Center (UAB) encompasses four care locations: the academic center in Birmingham and three satellite clinics (Montgomery, Opelika, Tuscaloosa). Unlike the academic center where providers are reliant on pediatricians to ensure adherence to recommended vaccines (with the exception of PPSV23), patients seen in the satellite clinics are provided with a prescription for PPSV23 and instructed to obtain it through their pediatrician. Data from prior studies suggest that pediatric patients with SCD may have lower vaccination rates than the general population. Thus, the purpose of this study was to determine adherence to recommended SCD-specific vaccination schedules among pediatric patients cared for at UAB , by: 1) determining the mean age at initiation of the HPV, MenACWY, Men B, and PPSV23 vaccines among UAB pediatric patients, compared with SCD-specific recommendations for initiation of each of these vaccines; and 2) comparing vaccination rates for HPV, MenACWY, and Men B among UAB pediatric patients age 13-17 years vs. general population peers.
Methods: We performed a retrospective chart review of clinic notes and the Alabama state vaccine registry (Immunization Patient Registry with Integrated Technology [ImmPRINT]) for records of UAB patients, to determine the age at vaccine initiation and number of doses received for HPV, MenACWY, Men B, and PPSV23 vaccines for all 870 UAB patients aged 3-22 years. We calculated the mean age at which the UAB patients initiated each vaccine, the proportion of patients completing each vaccine series, and (for PPSV23) whether patients were vaccinated at UAB or at another location. We used CDC published vaccination rates for HPV, MenACWY, and MenB from 13-17-year-olds in the general population (Pingali et al., MMWR Morb Wkly Rep 2021) to compare with rates from UAB patient peers.
Results: The 870 UAB patients were an average age of 12.1 ± 5.4 years; 51.5% were male and 33.9% received most of their care at satellite clinics.
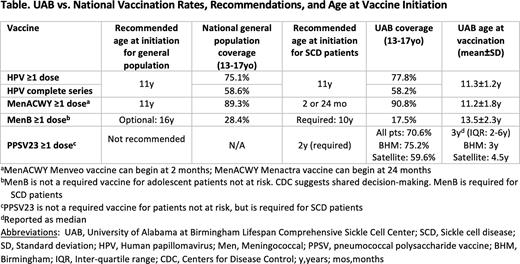
HPV vaccination: Mean ±SD age at HPV vaccine initiation was 11.3±1.2 years (recommended age at first dose=11 years, there are no SCD-specific guidelines for HPV vaccination); coverage for HPV vaccine series initiation and completion among 13-17-year-old UAB patients vs. general population peers was 77.8% vs. 75.1% and 58.2% vs. 58.6%, respectively (Table).
Meningococcal [MenACWY] vaccination: Mean±SD age at MenACWY initiation was 11.2±1.8 years (recommended age for patients with SCD is 2 months (MenACWY Menveo) or 24 months (MenACWY Menactra); recommended age for general population is 11 years). We found that patients with SCD were 32 (95% confidence interval [CI]: 22-47) times more likely to receive MenACWY after age 11 years.
Meningococcal B [MenB] vaccination: Mean age at MenB initiation was 13.5±2.3 years (recommended age for SCD patients is 10 years; vaccine is optional for general population); coverage for ≥1 MenB dose among 13-17-year-old UAB patients vs. general population peers was 17.5% vs. 28.4%, respectively.
PPSV23 vaccination: Overall PPSV23 coverage for UAB patients was 70.6%; patients seen at the academic medical center were 1.5 (95%CI, 1.1-2.0) times more likely to receive PPSV23 than patients seen at a satellite clinic (75.2% vs. 59.6%, respectively).
Conclusion: Adherence to SCD-specific vaccination age recommendations is poor. We found that patients with SCD were more likely to receive vaccines according to the general population vaccine schedule rather than the SCD-specific vaccine schedule. In addition, reliance on pediatricians to provide PPSV23 for satellite clinic patients resulted in lower PPSV23 vaccination rates among these patients. These data suggest a disparity in adherence to SCD-specific vaccination schedules and suggest the need to develop and test a healthcare provider-focused intervention aimed at improving adherence to the SCD-specific vaccination schedule for children with SCD.
Disclosures
Lebensburger:BPL: Consultancy; Novartis: Consultancy; Forma Therapeutics: Consultancy; Agios Pharmaceuticals: Consultancy. Landier:Merck Sharp & Dohme: Other: Wendy Landier's institution has received vaccine supply and laboratory services for an unrelated clinical trial through the Investigator Initiated Studies Program of Merck Sharp & Dohme (MISP #40083).
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal